Sanofi's Chlamydia Vaccine: A Step Closer To Approval With FDA Fast Track
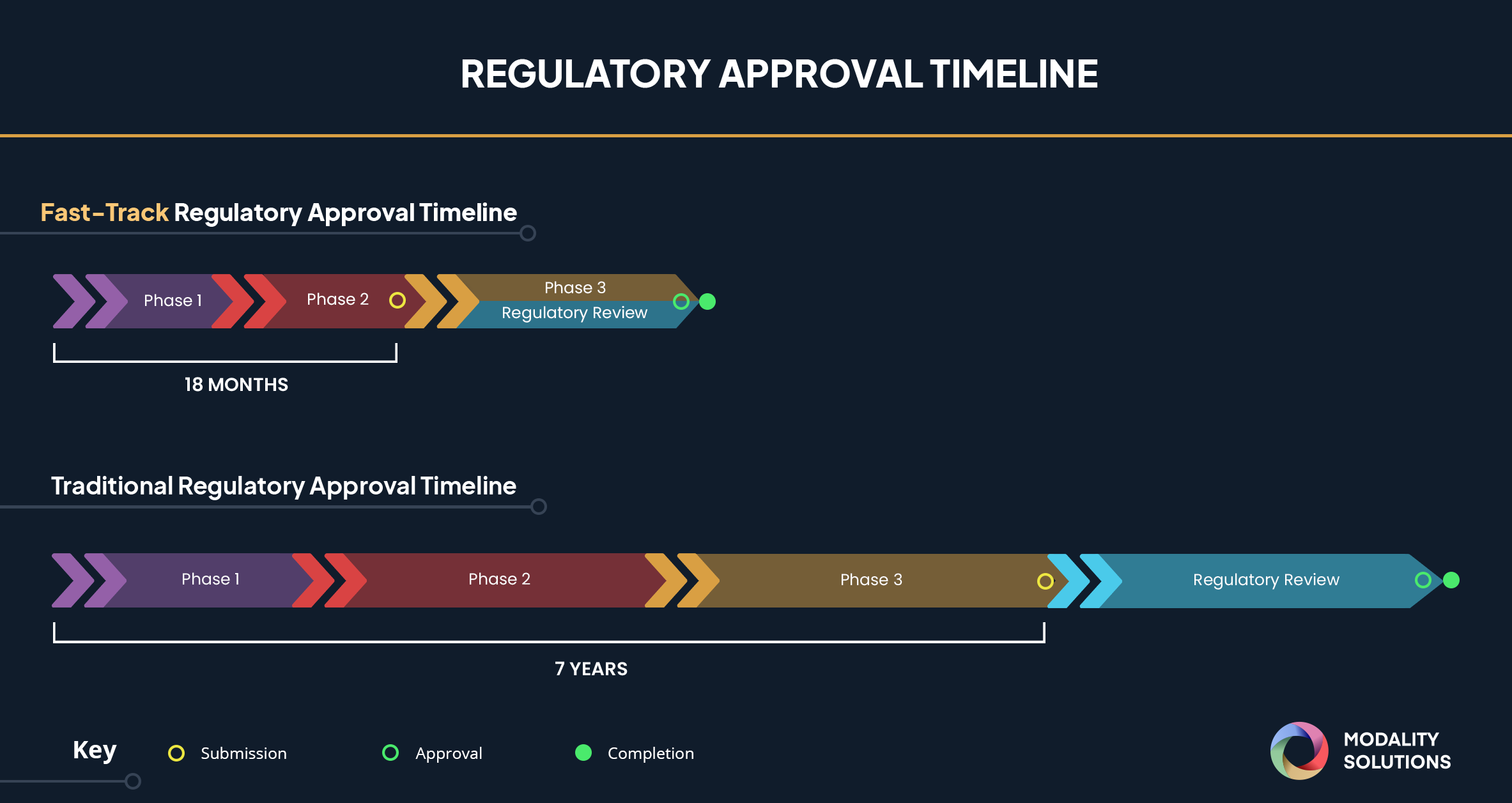
Table of Contents
FDA Fast Track Designation: What it Means for Sanofi's Chlamydia Vaccine
The FDA's Fast Track designation is a significant achievement for Sanofi's chlamydia vaccine. This program is designed to expedite the development and review of drugs and vaccines that address serious or life-threatening conditions with unmet medical needs. For Sanofi, this means a faster path to potentially bringing a chlamydia vaccine to market.
The expedited review process offers several key benefits:
- Faster clinical trial review: The FDA works closely with the company throughout the clinical trial process, providing guidance and feedback to streamline the process.
- More frequent meetings with FDA regulators: This allows for proactive problem-solving and ensures alignment on the development strategy.
- Potential for priority review and accelerated approval: Once the clinical trials are complete, the vaccine may receive priority review, further shortening the time to approval.
- Increased access to resources and guidance from the FDA: Sanofi benefits from increased support and expertise, potentially leading to a more efficient and effective development pathway.
For patients waiting for a chlamydia vaccine, the Fast Track designation translates to hope for a faster resolution to this critical public health issue. The accelerated development process offers the promise of earlier access to a preventative measure against a prevalent and potentially damaging infection.
The Science Behind Sanofi's Chlamydia Vaccine
While specific details regarding the precise mechanism of action for Sanofi's chlamydia vaccine remain limited pending full publication of clinical trial data, it's anticipated that the vaccine targets the Chlamydia trachomatis bacteria. The vaccine is likely to be protein-based, stimulating an immune response to key bacterial antigens.
The target population for Sanofi's chlamydia vaccine includes both men and women of reproductive age, the groups most commonly affected by chlamydia infections.
The efficacy and safety data from clinical trials are crucial. While comprehensive details await full publication, early indications of safety and the potential for an effective immune response have been positive enough to warrant FDA Fast Track designation. This suggests a promising profile for the vaccine, which should eventually be made available to a large section of the population.
- Immune Response: The vaccine aims to elicit both a humoral (antibody-mediated) and cellular immune response, neutralizing the bacteria and preventing infection.
- Vaccine Technology: The specific technology employed by Sanofi may involve innovative approaches like the use of adjuvants to enhance the immune response or targeted delivery systems.
- Clinical Trial Results: Although final data is pending, the success in receiving Fast Track designation indicates encouraging preliminary results concerning the vaccine's efficacy and acceptable safety profile. Further analysis of the clinical data will be crucial in evaluating the vaccine’s performance in a larger population.
Potential Impact on Public Health
Chlamydia is a significant global health concern. The World Health Organization (WHO) estimates millions of new infections annually, with many cases going undiagnosed and untreated. Untreated chlamydia can lead to serious long-term health consequences, including:
- Infertility: In women, chlamydia can cause pelvic inflammatory disease (PID), leading to scarring of the fallopian tubes and infertility.
- Ectopic Pregnancy: PID significantly increases the risk of ectopic pregnancy, a dangerous condition where the fertilized egg implants outside the uterus.
A successful chlamydia vaccine could dramatically reduce the global burden of chlamydia infections and their associated health complications.
- Chlamydia Prevalence: The high prevalence of chlamydia infections necessitates a preventative measure like a vaccine to curb transmission rates.
- Economic Burden: The costs associated with diagnosing, treating, and managing complications of chlamydia place a significant strain on healthcare systems worldwide. A vaccine could significantly reduce these costs.
- Reduced Hospitalizations: By preventing infections, the vaccine has the potential to reduce hospitalizations and long-term care associated with chlamydia-related complications.
Challenges and Future Outlook for Sanofi's Chlamydia Vaccine
Despite the positive development of FDA Fast Track designation, several challenges remain for Sanofi in bringing their chlamydia vaccine to market.
- Manufacturing Challenges: Scaling up vaccine production to meet global demand will require significant investment and logistical planning.
- Equitable Vaccine Access: Ensuring equitable distribution of the vaccine, especially to underserved populations, is crucial for maximizing its public health impact.
- Addressing Safety and Efficacy Concerns: Ongoing research and surveillance will be necessary to monitor vaccine safety and efficacy in diverse populations.
- Regulatory Hurdles: While Fast Track designation accelerates the process, regulatory approval still requires rigorous data review and approval, and the exact timeline for market availability remains uncertain.
The future success of Sanofi's chlamydia vaccine depends on continued research, development, and effective collaboration between Sanofi, regulatory agencies, and healthcare providers worldwide.
Conclusion
Sanofi's chlamydia vaccine has achieved a significant milestone with the FDA's Fast Track designation. This accelerates the development and potential approval process, offering substantial hope for reducing the global burden of chlamydia infections. The vaccine's innovative approach, coupled with the expedited review process, signifies a major step towards a healthier future. However, challenges remain in manufacturing, distribution, and ongoing research.
Call to action: Stay informed about the progress of Sanofi's chlamydia vaccine and the fight against this prevalent STI. Continue to follow updates on the development and potential approval of Sanofi's chlamydia vaccine to see how this game-changing innovation impacts public health.

Featured Posts
-
 How To Watch Giro D Italia Online Free And Legal Streaming
May 31, 2025
How To Watch Giro D Italia Online Free And Legal Streaming
May 31, 2025 -
 Bernard Keriks Wife Hala Matli And Family Details
May 31, 2025
Bernard Keriks Wife Hala Matli And Family Details
May 31, 2025 -
 Duncan Bannatynes Casablanca Trip Supporting Operation Smiles Childrens Hospital Work
May 31, 2025
Duncan Bannatynes Casablanca Trip Supporting Operation Smiles Childrens Hospital Work
May 31, 2025 -
 Faizan Zakis Triumph Winning The Scripps National Spelling Bee
May 31, 2025
Faizan Zakis Triumph Winning The Scripps National Spelling Bee
May 31, 2025 -
 American Universities Face Financial Challenges Amidst Changing Chinese Student Demographics
May 31, 2025
American Universities Face Financial Challenges Amidst Changing Chinese Student Demographics
May 31, 2025
