Strengthening US Vaccine Surveillance: A Response To The Measles Outbreak
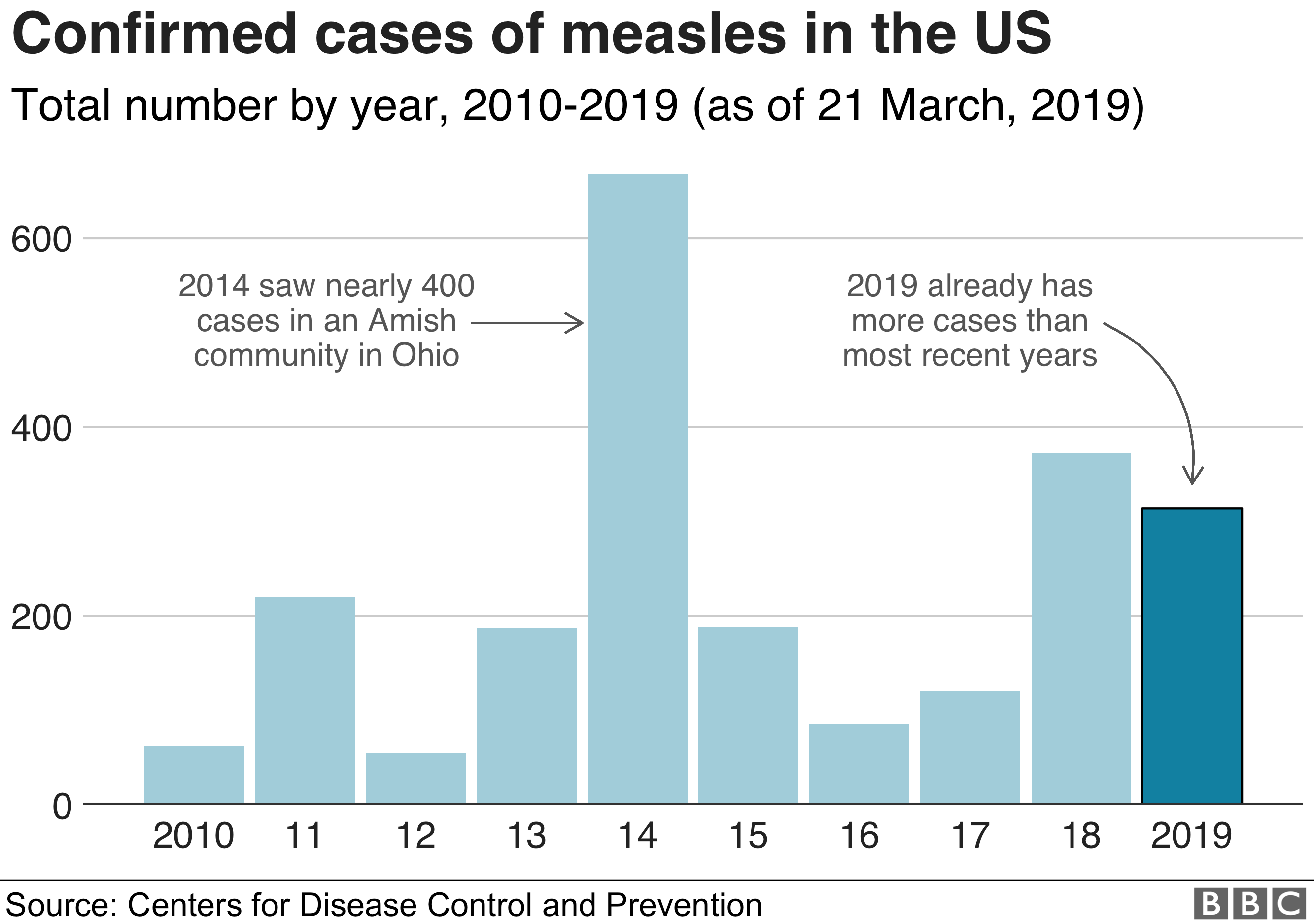
Table of Contents
The recent measles outbreak in the United States, with [Insert compelling statistic, e.g., "over 1,000 cases reported in 2023," or a specific state's high number of cases], serves as a stark reminder of the urgent need for improved vaccine surveillance. Robust vaccine surveillance systems are critical for preventing and controlling vaccine-preventable diseases (VPDs). These systems provide essential data to track vaccine coverage, identify outbreaks early, and guide public health interventions. Strengthening US vaccine surveillance is paramount to mitigating future outbreaks and protecting public health.
H2: Challenges in Current US Vaccine Surveillance Systems
The current US vaccine surveillance system faces significant challenges hindering its effectiveness. These challenges stem from data gaps, technological limitations, and public trust issues.
H3: Data Gaps and Inconsistent Reporting:
Current data collection methods suffer from inconsistencies across states and significant reporting delays. This is largely due to:
- Lack of standardized data collection protocols: Different states utilize varying methods, making national-level comparisons difficult and hindering accurate trend analysis.
- Reliance on passive surveillance: This relies on healthcare providers voluntarily reporting cases, leading to underreporting of both vaccine uptake and adverse events. For example, [State A] demonstrates a significantly higher reporting rate than [State B], potentially due to differences in incentives or reporting protocols. This disparity directly impacts the accuracy of national-level assessments of vaccine effectiveness.
- Underreporting of vaccine-related adverse events: Many healthcare providers do not report minor adverse events, leading to an incomplete picture of vaccine safety.
H3: Technological Limitations:
The lack of seamless integration between different health data systems hampers real-time monitoring and rapid response to outbreaks. Improvements are needed in:
- Electronic health record (EHR) integration: Standardized data formats and improved EHR interoperability are essential for efficient data extraction and analysis.
- User-friendly data entry systems for healthcare providers: Simplifying data entry will increase reporting rates and reduce errors.
- Investment in advanced data analytics: Modern data analytics tools are needed to analyze large datasets, identify trends, and predict outbreaks.
H3: Public Trust and Vaccine Hesitancy:
Vaccine hesitancy and misinformation significantly impact the accuracy of vaccine surveillance data. Addressing this requires:
- Strategies to improve public trust in vaccines and healthcare providers: Transparent communication and addressing public concerns are crucial.
- Addressing vaccine misinformation through effective public health campaigns: Counteracting misinformation requires targeted and evidence-based public health messages.
- The role of community engagement: Engaging trusted community leaders and healthcare providers can foster trust and encourage vaccine uptake.
H2: Strategies for Strengthening US Vaccine Surveillance
Addressing the challenges requires a multi-pronged approach focusing on enhanced data collection, technological advancements, and improved public health communication.
H3: Enhanced Data Collection and Reporting:
Improving the accuracy, timeliness, and completeness of vaccine surveillance data necessitates:
- Implementation of active surveillance methods: Proactively contacting healthcare providers and communities to gather data will increase reporting rates.
- Development of standardized data collection protocols: Uniform protocols across all states will ensure data consistency and comparability.
- Incentivizing healthcare providers for timely and accurate reporting: Financial incentives and recognition programs can motivate providers to prioritize reporting.
H3: Technological Advancements:
Technology plays a crucial role in modernizing vaccine surveillance:
- Leveraging EHR data: Extracting vaccination data from EHRs can significantly improve data completeness and timeliness.
- Integrating surveillance systems with disease outbreak prediction models: Real-time data analysis and prediction models can enable proactive interventions.
- Utilizing mobile health (mHealth) technologies for data collection and dissemination: Mobile apps can facilitate easy data entry and communication with healthcare providers and the public.
H3: Public Health Communication and Education:
Effective public health communication is key to addressing vaccine hesitancy:
- Targeted public health campaigns: Tailored campaigns addressing specific community concerns are more effective.
- Partnerships with community leaders and healthcare providers: Building trust through collaborations strengthens communication efforts.
- Utilization of social media and other communication channels: Reaching wider audiences through diverse platforms is crucial.
H2: Investing in a Robust Vaccine Surveillance System: The Economic Benefits
Investing in a robust vaccine surveillance system offers significant economic benefits.
H3: Cost-Effectiveness of Prevention:
Preventing outbreaks through improved vaccine surveillance is far more cost-effective than managing them:
- Reduced healthcare costs associated with treating VPDs: Early detection and prevention minimize the need for expensive treatments and hospitalizations.
- Decreased lost productivity due to illness: Preventing outbreaks reduces lost workdays and associated economic losses.
- Avoidance of costly outbreak control measures: Proactive surveillance minimizes the need for large-scale interventions like mass vaccination campaigns.
H3: Return on Investment (ROI):
The ROI of investing in improved vaccine surveillance is demonstrably positive: [Insert examples and quantifiable data from successful programs in other countries].
Conclusion:
The current US vaccine surveillance system faces significant challenges including data gaps, technological limitations, and public trust issues. However, by implementing enhanced data collection methods, leveraging technological advancements, and improving public health communication, we can significantly strengthen our vaccine surveillance capabilities. This will require increased funding, policy changes, and collaborative efforts across all levels of government and healthcare. We urge you to contact your representatives to advocate for improved funding and policies related to vaccine surveillance; investing in a robust vaccine surveillance system is not just a public health imperative but a fiscally responsible decision that promises a healthier future for all Americans. The strength of our nation's vaccine surveillance directly impacts our collective well-being.

Featured Posts
-
 Agents Earn A 1 500 Flight Credit Selling Paul Gauguin Cruises With Ponant
May 02, 2025
Agents Earn A 1 500 Flight Credit Selling Paul Gauguin Cruises With Ponant
May 02, 2025 -
 Fortnite Server Status Is Fortnite Down Right Now Chapter 6 Season 3
May 02, 2025
Fortnite Server Status Is Fortnite Down Right Now Chapter 6 Season 3
May 02, 2025 -
 Fortnite Server Status Is Fortnite Down Update 34 20 And Downtime
May 02, 2025
Fortnite Server Status Is Fortnite Down Update 34 20 And Downtime
May 02, 2025 -
 Clayton Keller 500 Nhl Points And A Missouri Legacy
May 02, 2025
Clayton Keller 500 Nhl Points And A Missouri Legacy
May 02, 2025 -
 Chris Columbuss Absence From Harry Potter And The Prisoner Of Azkaban A Directorial Change Explained
May 02, 2025
Chris Columbuss Absence From Harry Potter And The Prisoner Of Azkaban A Directorial Change Explained
May 02, 2025
